Acid-Base Disorders
Article Sections
Introduction
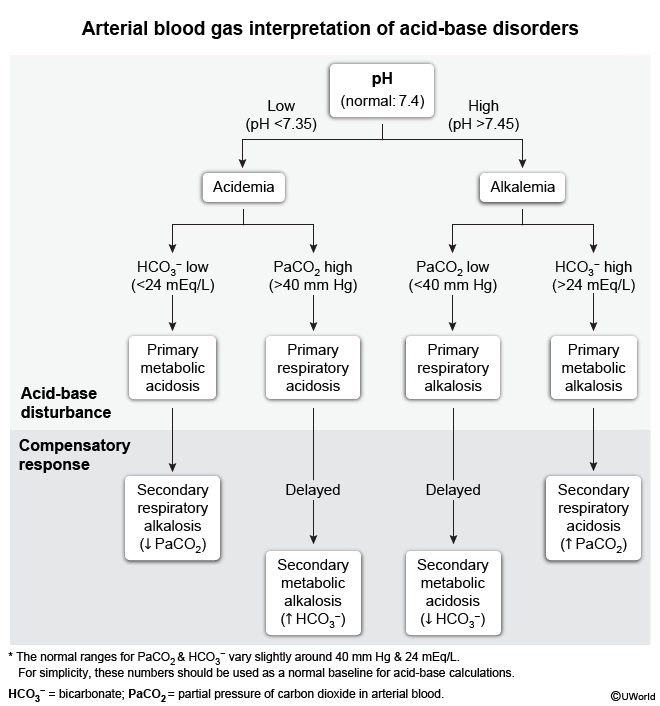
The maintenance of acid-base balance is crucial for normal cellular function and overall homeostasis. The body tightly regulates concentrations of hydrogen (H+), bicarbonate (HCO3−), and partial pressure of carbon dioxide (PaCO2) through buffering systems, respiratory control, and renal compensatory mechanisms. These systems work together to keep the blood pH between 7.35 and 7.45. However, various metabolic and respiratory insults can disrupt these regulatory mechanisms and result in acid-base disorders. These disorders are broadly categorized based on blood pH, with acidemia present for pH <7.35 and alkalemia for pH >7.45.
Although the terms are sometimes used loosely, metabolic or respiratory acidosis or alkalosis represent individual processes that contribute to the overall acid-base status, which should be described using acid
Continue Learning with UWorld
Get the full Acid-Base Disorders article plus rich visuals, real-world cases, and in-depth insights from medical experts, all available through the UWorld Medical Library.
Unlock Full AccessFigures